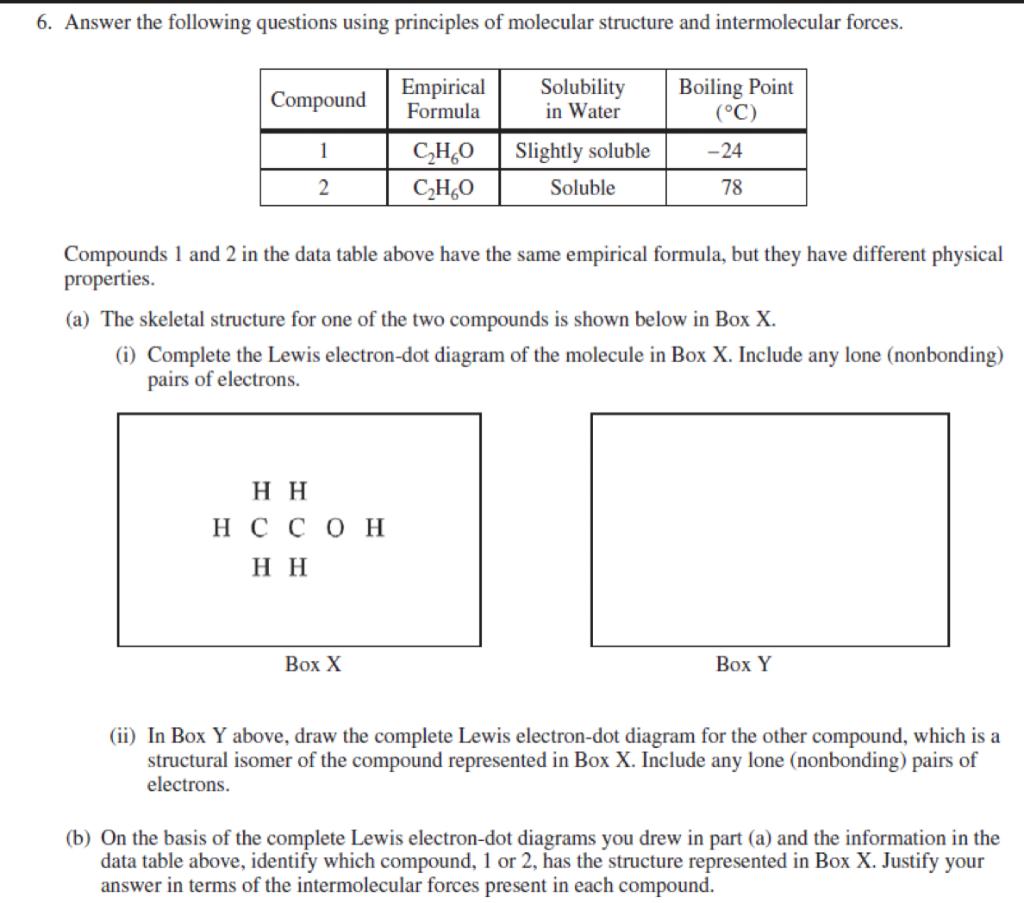
I Complete the Lewis electron dot diagram of the molecule in Box X Include any. A Draw an X above the peak that corresponds to the orbital with electrons that are on average closest to the nucleus.

Pre Test Big Idea 2 Chapters 8 9 10 Pdf Free Download
B Which of the four molecules contains the shortest carbon-to-carbon bond.
. Draw the complete Lewis structure for the condensed structure NCCH2COH in the sketch box below. Then determine the electron pair geometry EPG make a 3-dimensional representation drawing and determine the molecular geometry MG for all of them. You need to use a pen or even a dotting Device to obtain this final result.
You may need a little something to produce modest dots with. Make 5 dots so that they connect. Ii In Box Y below draw the complete Lewis electron-dot diagram for the other compound which is a structural isomer of the compound represented in Box X.
1 point is earned for a correct Lewis diagram. Up to 24 cash back I point is earned for a correct Lewis diagram. Be sure to add all lone pairs.
Angle x is approximately 120. Be sure to add all lone pairs. Course Title CHE 115.
B Based on the spectrum write the complete electron configuration of the element. Here we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Complete the following table with a name or.
The carbon atoms in the molecule are labeled x and y respectively. Formula Lewis structure EPG 3-D drawing MG polar. In the box below draw the complete Lewis electron-dot diagram of N 2.
The complete photoelectron spectrum of an unknown element is given above. Draw the complete Lewis structure for and indicate all of the bond angles for each of the following compoundsa benzeneb cis-2-butenec 12-dichloroethanePS28a. Examples for Drawing Lewis Dot Structure for Covalent Bonds.
B On the basis of the Lewis electron-dot diagram that you drew in part a predict the molecular geometry of the IF 3 molecule. H C N H O H x y i In the molecule angle x is not 180. In Box Y Above Draw The Complete Lewis.
In the box below draw a complete Lewis electron-dot diagram for a molecule of ClF 3. Trigonal Pyramidal PCl 3 gCl 2 g PCl 5 g Kc011 A 060mol sample of PCl 3 g and a 070mol sample of Cl 2 g are placed in a previously evacuated 10L rigid container and the reaction represented above takes place. Methanamide CH3NO is a liquid at 250C.
I complete the lewis electron dot diagram of the. C A Lewis electron-dot diagram of a molecule of ethanoic acid is given below. Justify in terms of bond.
In box y above draw the complete lewis As being a starter you can make your very own Nail Artwork Effect working with two strategies. 1 point is earned for a correct Lewis diagram. Visit the College Board on the Web.
Up to 24 cash back Box X ii In Box Y below draw the complete Lewis electron-dot diagram for the other compound which is a structural isomer of the compound represented in Box X. Up to 24 cash back a Complete the Lewis electron-dot diagram of methyl methanoate in the box below. Include any lone nonbonding pairs of electrons.
Two possible geometric shapes for the ClF 3 molecule are trigonal planar and T-shaped. What are structural isomersb Draw and name all of the structural isomers for each of the following compoundsi C7H16ii C5H11ClPS29. Draw the complete Lewis electron-dot diagram of N2.
Estimate the observed angle. Answer the following questions related to HF and the titration of HF aq a In the following box draw the complete Lewis electron- dot diagram of the HF molecule. The student does some research and learns that the molecule has a dipole moment.
Acquiring 3D decorations comprised of plastic and glue them on the nail or you may get your personal acrylic and paint your very own nailsSome state that it needs a lot of expertise on painting your own. A In the box provided below draw a complete Lewis electron-dot diagram for the IF3 molecule b On the basis of the Lewis electron-dot diagram that you drew in part a predict the molecular geometry of the IF3 molecule. In the Lewis-dot structure the valance electrons are shown by dot.
A Draw the complete Lewis electron-dot diagram for ethyne in the appropriate cell in the table above. Draw the complete Lewis Structure for each. See the following Lewis dot structure diagrams for a few covalent compounds.
Which of the shapes is consistent with the fact that the ClF 3 molecule has a dipole moment. Located directly above the lowest part minimum of the potential energy curve. 0 2013 The College Board.
Therefore the total number of valence electrons in 26 26 24. Then top rated it off with a yellow dot at the center. Answer the following questions about N 2 and N 2 H 4.
Chemistry questions and answers. Reference the How to Draw a Lewis Dot Structure for a Step by Step guide. H a The complete Lewis electron-dot diagram for methanamide is shown below.
A In the box provided below draw a complete Lewis electron-dot diagram for the IF 3 molecule. Include any lone nonbonding pairs of electrons. A student performed a titration 1 answer Top answer.
Three electron domains around the carbon atom will maximally separate the electrons and minimize the energy when. H 1 point is earned for a correct Lewis diagram. One point is earned for a correct Lewis diagram can be done with dots or lines.
C In the S02 molecule both of the bonds between sulfur and oxygen have the same length. Check answers below Recall. What are structural isomersb Draw and name all of the structural isomers for each of the following compoundsi C7H16ii C5H11ClPS29.
B What is the shape of the PCl3 molecule. Up to 24 cash back a The complete Lewis electron-dot diagram for methanamide is shown below. According to Lewis-dot structure there are 10 number of bonding electrons and 14 number of non-bonding electrons.
Draw the complete Lewis structure for and indicate all of the bond angles for each of the following compoundsa benzeneb cis-2-butenec 12-dichloroethanePS28a. Ve 10 Re 10 - 4 6 3 more pairs to use. The lowest potential.
Pages 5 This preview shows page 4 - 5 out of 5 pages. In box y above draw the complete lewis. A In the box below draw the complete Lewis electron-dot diagram for PCl 3.
Show all valence c electrons. A In the following box draw the complete Lewis electron-dot diagram of the HF molecule. Determine whether each molecule is polar or non-polar.
Estimate the observed angle. This nail art is great for the spring and summer. H H Box X ii In Box Y below draw the complete Lewis electron-dot diagram for the other compound which is a süuctural isomer of the compound represented in Box X.
So is i In the molecule angle x is not 1800. The given molecule is As we know that sulfur and oxygen has 6 valence electrons. Justify your answer in terms of Coulombs law.
0 2013 The College Board. In the box below draw the complete Lewis electron-dot. 1 Arrhenius definition - proton transfer occurs from the acid to base.
C ору P ChemDoodle. B 1 IC x X 5 ο Ω Ξ 11 010000 Word Limit HF aq OH aq F aq H20 1 A student performed a titration of HF aq with NaOH aq. The acid-base reaction can be defined as follows.
Draw the complete Lewis structure for the condensed structure NCCH2COH in the sketch box below. H I point is earned for a correct Lewis diagram. Include any lone nonbonding pairs of electrons.

Solved 6 Answer The Following Questions Using Principles Of Chegg Com

0 comments
Post a Comment